Method Development for Biologics
Biosimilar refers to the biological drug that is highly similar to the available marketed drug. The patents on the first generation of licensed biological drugs have either expired or will expire soon, allowing biosimilar to enter the market. Biosimilar allows greater accessibility and cost-effectiveness of the biological therapy to the patients. Biological drugs are made by complex mechanisms involving living organisms, as opposed to small molecules, which are made by chemical syntheses. Because of their structural complexity and safety risk, replicating protein molecules is a much more challenging task. This is extremely crucial because the immunogenicity of biological drugs has attracted a lot of attention as a safety concern in recent years, reinforcing the need for extensive monitoring prior to clearance and a long duration of post-marketing supervision.
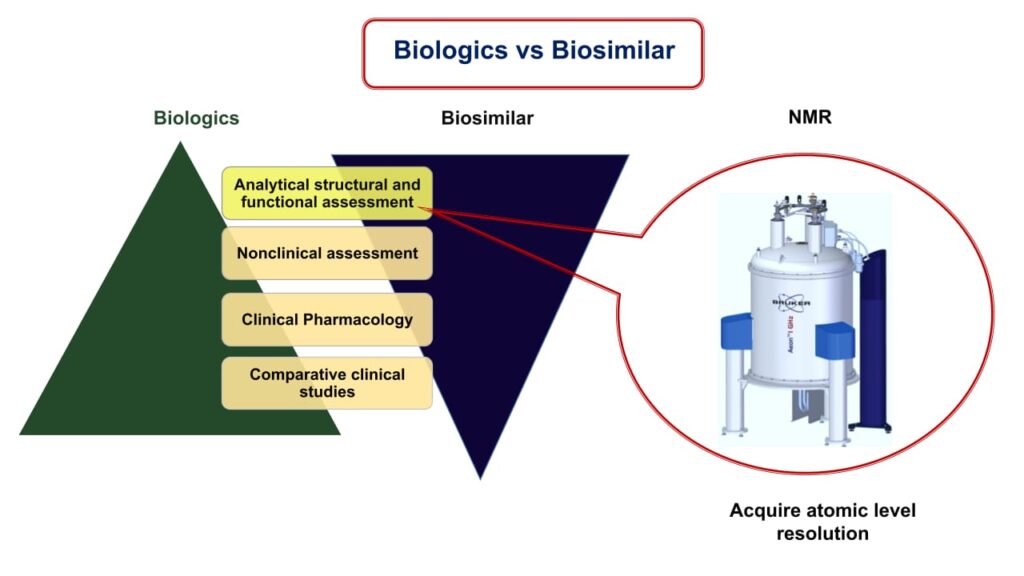
Nuclear Magnetic Resonance (NMR) can give atomic level resolution inside the molecule which can significantly identify the small changes in the chemical environment around the individual nuclei. The comparison of the NMR spectra will give clear idea about the structural similarity between the reference and the new drug product. It can be used to derive the structural information ranges from very small molecule to large biopharmaceuticals such as monoclonal antibody. In our lab we aim to develop robust NMR methods to study Biosimilarity and characterization of different biopharmaceuticals.
Team: Soumyaranjan Pujahari