BB525 Biological Thermodynamics and Kinetics
Varsha Semester : July to November
Credit structure: 2 (L), 1 (T), 0 (P), 3 (C)) Half-semester
BB525 Biological Thermodynamics and Kinetics
Thermodynamic functions – U, A, H, S and G. The First law: work, heat, energy, heat transactions, enthalpy, standard enthalpy changes. The Second law: entropy, entropy changes accompanying specific processes. The Third law and Biology. Chemical equilibrium: Gibb’s energy minimum, description of equilibrium, How equilibria respond to pressure, temperature & pH. Applications of thermodynamic principles to biological systems. Statistical thermodynamics: distribution of molecular states (introduce molecular partition function), the internal & the statistical entropy, Boltzmann distribution. Basic kinetic concepts: Reaction stoichiometry, rates of consumption & formation, extent of reaction, rate of reaction, Analysis of kinetic results, influence of temperature on reaction rates. Theories of reaction rates: Kinetic theory of collision, transition state theory of reaction rates, potential energy surfaces and reaction dynamics; diffusion; kinetics of unimolecular and bimolecular reactions; application of kinetics to biological systems. Catalysis: General catalytic mechanism (Arrhenius intermediate, Van’t Hoff intermediate), Acid-base catalysis, acidity function, Enzyme catalysis, Michaelis-Menten equation, Inhibition, effects of pH, Bisubstrate reactions (sequential reaction, ping-pong reactions).
Texts / References:
1. Biothermodynamics : the study of biochemical processes at equilibrium, Edsall, J.T., Gutfreund, H., Chichester : John Wiley, 1983
2. Biological thermodynamics, Haynie, D.T. Cambridge: Cambridge University Press, 2001
3. Thermodynamics and kinetics for the biological sciences Hammes, G.G. J Wiley 2000
4. Atkin’s Physical chemistry. 8th ed. P W Atkins & J. dePaula, Oxford Univ Press, 200
BB526 Biomolecular Spectroscopy
Vasant Semester : January to April
Credit structure: 2 (L), 1 (T), 0 (P), 3 (C)) Half-semester
BB526 Biomolecular Spectroscopy
Content : UV-visible absorption spectroscopy: Beer-Lambert’s law; applications of UV-visible difference spectroscopy; Circular dichroism in protein analysis; Fluorescence spectroscopy of Biomolecules: Jablonsky diagram; quantum yield, static and dynamic quenching of fluorescence, energy transfer, polarization, anisotropy, time-resolved fluorescence; FT-IR spectroscopy, Nuclear Magnetic Resonance Spectroscopy: chemical shifts, coupling constants, ring currents, paramagnetic shifts, spin-spin and spin-lattice relaxation times, NOE, chemical exchange; Mass spectrometry of biomolecules.
Texts / References:
1. C.R. Cantor & P.R. Schimmel; Biophysical Chemistry, Part-2. W.H.Freeman & Co. 1980.
2. J.R. Lalcowicz; Principles of Fluorescence Spectroscopy. Plenum Press.
3. J.D. Campbell & RA. Dwek; Biological Spectroscopv. Benjamin, 1984.
4. P.S.C Mathews; Quantum Chemistry of Atoms and Molecules. Cambridge University Press, 1986
BB 645: Drug Discovery and Development
Varsha Semester :July to November
Credit structure: 3 (L), 0 (T), 0 (P), 3 (C)) Half-semester
BB 645: Drug Discovery and Development
Introduction to drug design and discovery, Molecular Recognition, Ligand-based drug design, Bio-structure based drug design, Drug-Like Properties and Decision Making in Medicinal Chemistry, peptide, and Protein Drug Design, Enzyme Inhibitors: Biostructure- and Mechanism-Based Design, Antibiotics, Principles of drug absorption, drug metabolism, and distribution – intestinal absorption, metabolic stability, Global Regulatory Affairs and different steps involved, Regulatory Objectives.
Text/References:
1. Krogsgaard-Larsen et al. Textbook of Drug Design and Discovery. 4th Edition. CRC Press
2. Nally, J. D. (2006) GMP for Pharmaceuticals. 6th edition. CRC Press 4. Brody, T. (2016) Clinical Trials: Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines. Academic Press.
BB 617: Biophysical Chemistry
Conformation of Biological Macromolecules, Structure or proteins, Structure of Nucleic acids, Lipids, Conformational analysis and forces that determine protein and nucleic acid structure, Ligand – Macromolecular interactions, Protein folding, Computer applications in Biology. Spectroscopic methods, Adsorption spectroscopy, Difference, and derivative Spectroscopy as applied to biomolecular interactions, Fluorescence spectroscopy, Membrane Proteins, Nucleic acid probes, Fluidity, Polarity, and Viscosity.
Text/References:
1. Physical Biochemistry – Applications to biochemistry and molecular biology, D. Freifelder: W.H. Freeman and Co., 1986.
2. Biophysical Chemistry (Part 1-3), C. R. Cantor and P. R. Schimmel; W.H. Freeman and Co, 1980.
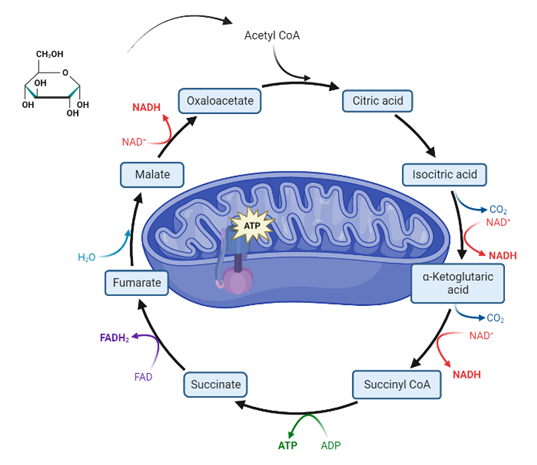
BB 404 and BB 404(M): Metabolism and Bioenergetics
Semester :Full Semester
Credit structure: 3 (L), 0 (T), 0 (P), 6 (C)
BB 404 and BB 404(M): Metabolism and Bioenergetics
Overview of metabolism; the concept of the flow of matter and energy; thermodynamics of coupled systems and non-equilibrium reactions; Biological energy currencies: high energy bond, reducing power and interconversions of energy forms; classification of a living system based on carbon and energy requirements; methods to study metabolism; carbohydrate and lipid catabolism; glycolysis; TCA cycle; fatty acid oxidation, other metabolic routes of carbon; oxidative phosphorylation; Calvin cycle and other avenues of harvesting light energy; gluconeogenesis; glycogen metabolism; integration of metabolism and concepts of metabolic regulation.
Text/References:
1. Metabolic Regulation, R.S. Ochs, R.W. Hanson, and J. Halls: Elsevier, 1985.
2. Physical Chemistry, P.W. Atkins; ELBS, 1981.
3. Lehninger Principles of Biochemistry, D.L.Nelson, and M.M.Cox. 4th ed. W.H.Freeman, 2004.
4. Bioenergetics. D.G.Nicholls and S.J.Ferguson, 2nd ed. Academic Press, 2002.
BB640: Biologics and Biosimilars
Semester: Half-semester
Credit structure: 3 (L), 0 (T), 0 (P), 6 (C))
BB640: Biologics and Biosimilars
Introduction to Biologics and Biosimilars, Development and its role in Therapeutics, Cell Line Development and Upstream Bioprocessing, cell culture methods, clone selection, and optimization, Bioreactors, Scale-up optimization Critical Quality Attributes for Biologics and Bio-similars, Glycosylation, De-amidation, Charge Variant analysis, Analytical Methods to measure Glycosylation, De-amidation, Charge Variant (AUC, CE, Mass spectrometry) Biophysical and analytical Characterization of Biologics products, Primary, secondary and tertiary structural analysis by various Biophysics Methods, Protein aggregation principle, and analysis by DLS and SE-HPLC, Thermodynamic stability by DSC, ITC Downstream Purification for Biologics, Regulatory Approach for Biosimilars, Globalization of Biosimilars
Text/References
1. Introduction to Biologic and Biosimilar Product Development and Analysis, Nagel, Karen M. Springer, 2018
2. Biosimilars: Regulatory, Clinical, and Biopharmaceutical Development Editors: Gutka, Hiten J., Yang, Harry, Kakar, Shefali (Eds.) Springer, 2018

BB 657 : Regulatory Aspects of
Drug Development
Credit Structure: 3(L), 0(T), 0(P), 3(C)
Dates: 2 lectures per week
(5th of Jan to 16th Feb 2024) Time: 2 hours each
(Wed/Fri: 3:30-5:00 pm)
Course Instructors:Professor Ashutosh Kumar and Dr. Narendra Chirmule
BB 657 : Regulatory Aspects of Drug Development
Course Description:Drug development is at a turning point in human medicine. Over the past three decades, the development of biotherapeutics has revolutionized innovation in medicines. Efficiency and Quality Compliance are critical to achieving innovation and affordability. This course will provide an overview of the multi-dimensional nature of drug development, which involves regulatory, new technologies, statistical, and quality considerations. This 6-week course will introduce the concepts of drug development, which include, pharmacology, toxicology, product development, clinical trials. All of these topics will be addressed based on regulatory requirements by the FDA. Risk assessment and mitigation will be discussed using a role-play process. The content of the course includes seminars, case studies, project reports, and journal article-reviews.
Text/Reference:
Biophramacheutical Drug Design and Development by Susanna Wu-Pong and Yon Rojanasakaul Second Edition Humanna Press SBN: 978-1-59745-532-92. Introduction to Biologic and Biosimilar Product Development and Analysis by Karen M. Nagel Springer, ISBN: 978-3-319-98428-53. https://www.sciencedirect.com/science/article/pii/B97880406690000674. Social Aspects of Drug Discovery, Development and Commercialization https://doi.org/10.1016/B978-0-12-802220-7.00001-6
DH 804 : Magnetic Resonance Imaging
Credit Structure: 3(L), 0 (T), 0(P), 3(C)
Half Semester Course (Second Half)
Course Instructors:Professor Ashutosh Kumar and Prof. NR Jagannathan, Distinguished Visiting Professor KCDH
DH 804 Magnetic Resonance Imaging: From Physics to Physiology
Course Description:
Basic: 1. An Overview of Magnetic Resonance Imaging, Electricity and Magnetism ;2. Nuclear Magnetism, Equilibrium-Saturation The Magnetic Resonance Image;3. Radiofrequency Pulse Sequences, Magnetic Resonance Imaging Tissue Parameters;4. Manipulating Magnetic Resonance Image Contrast, Fourier Transforms in Magnetic Resonance Imaging The Imaging System; 5. Magnetic Resonance Imaging Hardware;6. Primary Magnetic Resonance Imaging Magnets, Image Formation;7. Digital Imaging, A Walk Through the Spatial Frequency Domain;8. Magnetic Resonance Images
Pulse Sequences;9. Spin Echo Imaging, Chemical Shift and Magnetization Transfer;10. Steady State Gradient Echo Imaging, Echo Planar Imaging
Applications;11. Nuclear Magnetic Resonance Spectroscopy, Partially Parallel Magnetic Resonance Imaging 12. Magnetic Resonance Angiography, Perfusion Imaging and safety consideration.
Text/Reference:
1. 302223Magnetic Resonance Imaging: Physical and Biological Principles302224 by Stewart C. Bushong. ISBN:3022409780323073547
- Clinical Magnetic Resonance Imaging302224 by Edelman, Hesselink and Zlatkin. Publisher : 302240Saunders; 3rd edition (16 November 2005), ISBN-10 : 3022400721603068
- Magnetic Resonance Imaging 302226 Physical Principles and Sequence Design302224 by Haacke, Brown, ISBN:9780471720850
- Spin Dynamics: Basics of Nuclear Magnetic Resonance302224 by Malcolm H. Levitt. ISBN: 978-0-470-51117-6