FROM PHYSICS TO PHYSIOLOGY AT THE MEMBRANE-MOTOR INTERFACE
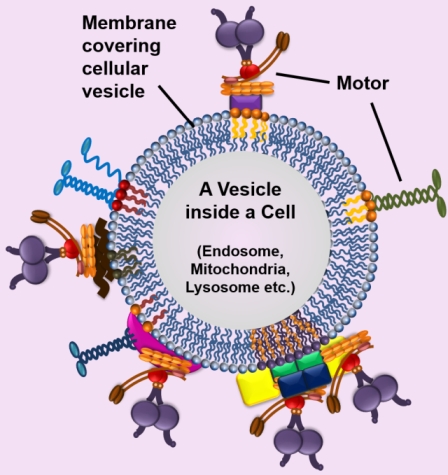
The above title of a Commentary written by us is perhaps the best way to summarize our research. Motor proteins will almost always assemble on a lipid membrane inside cells before they do anything. We explore this Membrane-Motor interface at different levels of complexity. This Sketch shows the experimental approaches that we use. To us the Membrane-Motor interface is a dynamic entity that responds to cellular and metabolic demands, thereby harnessing the force from motors to control many cellular processes relevant to health and physiology. This membrane can be a phospholipid bilayer (for example, the membrane covering an endosome as depicted in figure to the right) or a phospholipid monolayer (covers a fat-containing Lipid Droplet; not shown here). How this difference (monolayer versus bilayer) makes a difference is a central theme in our research. To find out more, see below.
We are also interested in Drug induced Liver injury, Peptide-lipid binding and related fields. Write to us if you have any ideas on the same in relation to our work.
LIPID HOMEOSTASIS – WHAT YOUR LIVER DOES WHEN YOU SLEEP
Kumar et.al. Journal of Cell Biology 2019 ; Rai et.al. PNAS 2017 ; Sadh et. al. PLoS ONE 2017 ; Barak et. al. Nature Methods 2013 ;
Kamerkar et al PNAS 2022 ; Popular article in The Biochemist- Who Let the Fat Out?
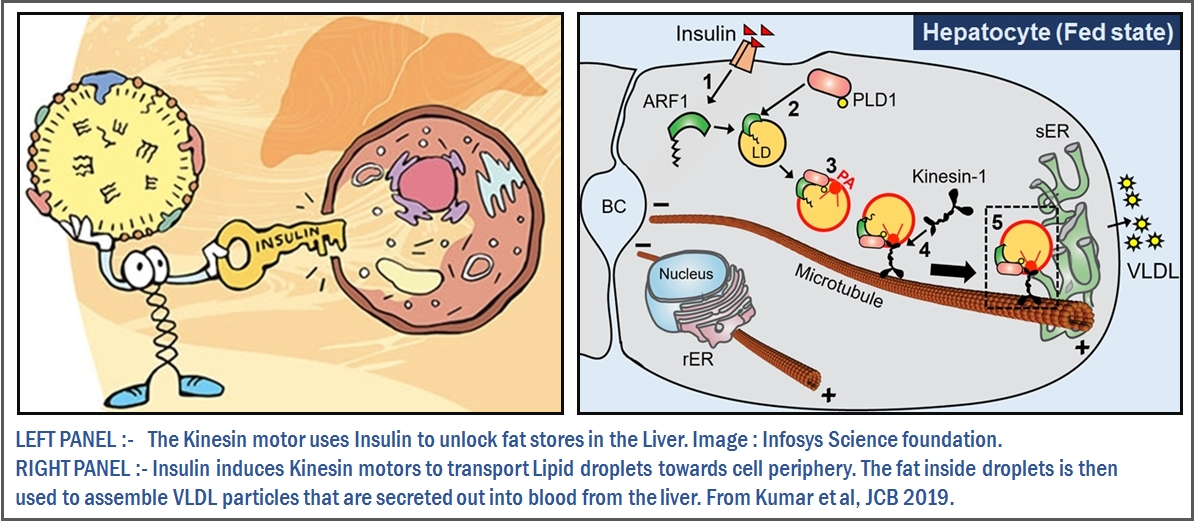
Excess fat circulating around in blood means obesity, diabetes and heart disease. We recently found a mechanism that allows the liver to control fat secretion into blood. When we fast, for example we go to bed after dinner, glucose is in plenty but soon it runs out and the body must burn fat to keep going. Fat-containing tissues respond by sending off fat to the liver, which gets bloated with fat, but still continues to secrete this fat at a constant rate. This remarkable elasticity of the liver prevents dangerous fat overload in the blood. But, how does all this work at the level of cells and molecules?
We showed that insulin recruits the motor protein kinesin to fat bodies (see image on right). Kinesin propels fat to the periphery of liver cells, from where the fat is secreted out in the form of VLDL. As the night wears on, fat accumulates in the liver, but insulin levels also diminish. Kinesin now falls off from the fat bodies, secretion is toned down and systemic fat homeostasis can be achieved. So, you should probably thank your liver when you get up fresh as a flower tomorrow morning.
TRANSPORT AND DEGRADATION OF PHAGOSOMES
Sanghavi et.al. PNAS 2021 ; Sanghavi et.al. Curr Biol 2018 ; Rai et.al. Cell 2016 ; Rai et.al. Cell 2013 ; Soppina et.al. PNAS 2009
Popular article in The Scientist
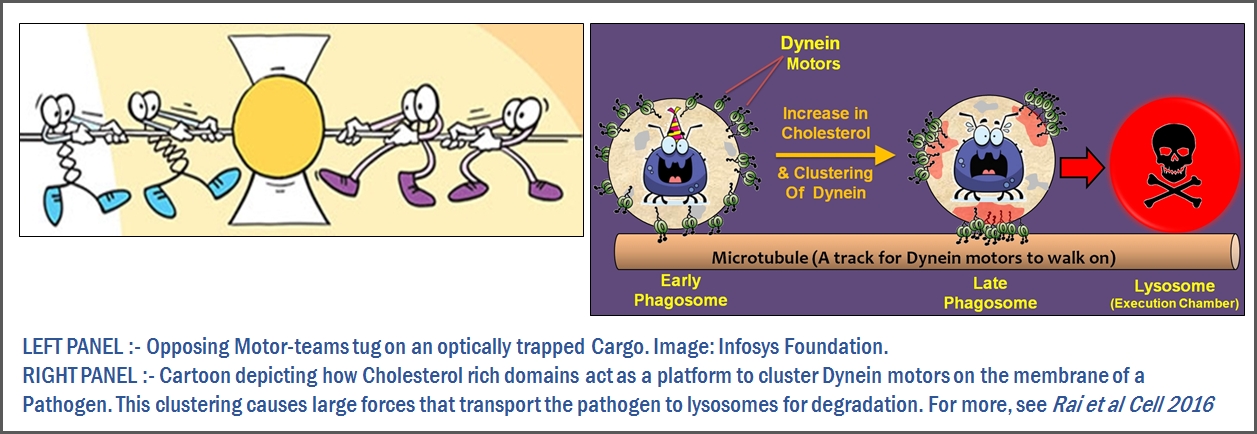 To keep you healthy, your immune cells must constantly ingest and then degrade a variety of pathogens (bacteria, parasites etc). Once ingested, pathogens are enclosed in a microscopic chamber called a “Phagosome”. Now, it is a game of cat-and-mouse. Will the bug be degraded, or will it fool the immune system to make you sick? Under normal circumstances, motor proteins will transport the phagosome to an acidic chamber called the lysosome, where the pathogen can be degraded. However, many pathogens can actually hijack the motor proteins to avoid getting degraded.
To keep you healthy, your immune cells must constantly ingest and then degrade a variety of pathogens (bacteria, parasites etc). Once ingested, pathogens are enclosed in a microscopic chamber called a “Phagosome”. Now, it is a game of cat-and-mouse. Will the bug be degraded, or will it fool the immune system to make you sick? Under normal circumstances, motor proteins will transport the phagosome to an acidic chamber called the lysosome, where the pathogen can be degraded. However, many pathogens can actually hijack the motor proteins to avoid getting degraded.
Along with the cell biology, we are interested in the physical processes that decide the fate of a phagosome (and the pathogen inside it). For example, see the above image where we found a “Tug-of-war” that decides where the phagosome will end up. We demonstrated how cholesterol dictates dynein function during phagosome maturation. The geometrical organization of dynein on a phagosome’s lipid membrane was modified by clustering dynein into cholesterol-rich lipid rafts (see figure). This dramatically altered dynein’s cooperative function with no apparent effect on it’s single molecule properties. These results appear relevant to immune invasion by Mycobacteriumm Salmonella, Leishmania. We also showed that the activity of opposing kinesin and dynein teams on a phagosome can be modelled as the tossing of a coin.