Molecular motors – A lesson in Nanotechnology from Nature (Roop Mallik)
They are small, and there are billions of them inside you. Tiny machines, a thousandth of the thickness of human hair, but robust and designed for an amazing variety of functions. Science fiction? Think again this is real, as real as flesh and blood !! If you can get your hands on a high school biology text book, flip through to the mandatory schematic of an animal cell. Look closely, what you will see is not a floppy bag with random things thrown in here and there. There is amazing structural organization within the cell, with several compartments (e.g. the nucleus, Golgi bodies, mitochondria) at specific locations. Many of these compartments are specialized factories, each with its own assembly line requiring specific raw material as input and generating specific products. A constant give-and-take of materials occurs between these factories inside the cell, because each factory is dependent on the other to function. In the big picture of things this incessant exchange of material keeps the factories of the cell functioning, which in turn is what keeps us alive.
FIGURE :- How things move around inside a Cell Simplified schematic of the transport network of a typical interphase cell. Molecular motors are shown ferrying different cargoes. Kinesin motors usually move towards the cell periphery, while Dyneins move towards the cell centre. More detailed schematics of the motors show the “legs” (some people call them motor-heads!!) which make contact with the microtubule filaments. Actin-based motors (Myosins) and the actin filaments are not shown. For an idea of the size scales involved, Kinesins and Dyneins have dimensions of 50-100 nanometers. A nanometer is one-billionth of a meter. The motors exert forces of the order of picoNewtons. One picoNewton is a thousand-billion times smaller than a Newton. We exert forces of the order of tens of Newtons in daily life.
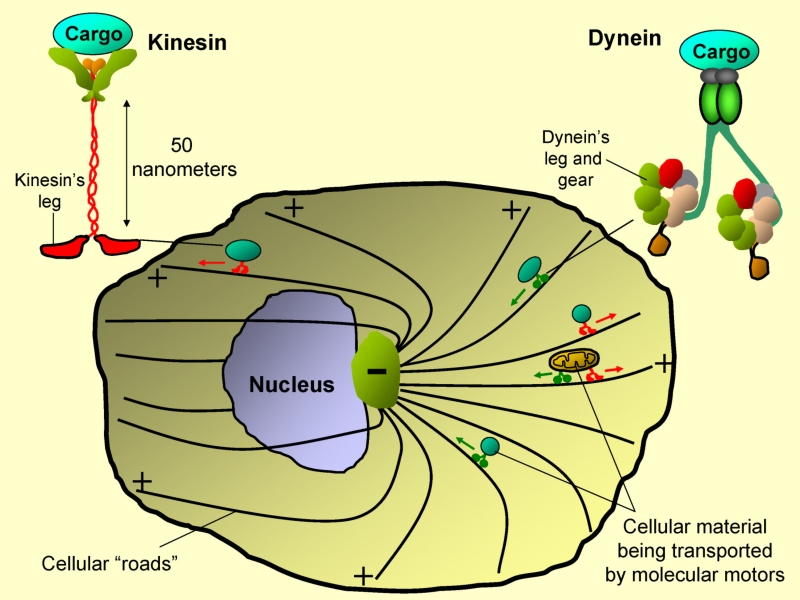
This flow of material occurs in a highly regulated, so that the right things are present at the right place and time. How does this transport of materials take place? This is where the army of tiny machines called molecular motors comes in. The cell has a network of roadways (one kind of roadways are called microtubules, see Figure) with heavy traffic of molecular motors on them. These motors can be thought of as porters ferrying all kinds of material within the cell. You will be surprised at how well this analogy of molecular motors with porters works, but dont forget that these motors are a 10-million times scaled down version of what your idea of a porter is.
So, what exactly is this motor that works on a molecular scale? One example is a protein with two legs walking along on the cellular roadway, stepping just like a porter and carrying some cellular material as cargo. During every step that the motor takes, it has to generate force and therefore does some work. The energy required for this comes from chewing up a molecule called ATP, which has energy stored in its chemical bonds. For every step that the molecular porter takes, it needs one little packet of energy in the form of an ATP molecule. So, if you travel inside the cell and need somebody to carry your bags, make sure you give them a constant supply of ATP. Just three meals a day does not work at the molecular scale.
Kinesin, one of the best studied molecular motors walks with precise steps of 8 nanometers. For each step, kinesin uses one molecule of ATP and generates 6 piconewtons of force. A simple calculation shows that this makes kinesin a nano-machine with almost 50% efficiency, which is comparable to many machines of human design. To give you an idea of the magnitude of scales here, if a kinesin motor walked upwards starting at your toenail, it would take about 100 million steps to reach your nose. Approximately 1 million-million kinesins would have to team up together to arm-wrestle with you and have any hope of winning. We are really talking nanotech here.
For anyone impressed with kinesin, there is more to come. Dynein is a second class of motors ferrying cargo within the cell, and is much more complicated than kinesin. There is evidence (Mallik et al, Nature 2004) that nature has implemented a nanoscale gear mechanism within this complexity. It appears that dynein normally walks with a step size about thrice the size of kinesin. However, if you pull dynein backward the motor can shorten its stepsize and resultantly produce more force, which is just like shifting gears in your car on an uphill road. Only future research can tell why nature felt the need to implement such complex architecture at these minute size scales.
There are many other classes of motors which I have not discussed here for reasons of space, and also to keep the discussion simple. One common theme that has emerged from years of research is that of surprisingly intricate and robust architecture within these molecular motors at a size scale which we are only now beginning to comprehend. It is these little things in life that matter, so let us congratulate nature on a little job very well done !!
LIPIDS INDUCE GEOMETRICAL REORGANIZATION OF MOTORS FOR PATHOGEN DEGRADATION
Molecular motors with inclination to move in opposite direction (e.g. Kinesin and Dynein) are simultaneously present on a cargo inside the cell. Infact, multiple motors of each class are often present simultaneously on a cargo. Do the motors work against each other (tug-of-war model), or is their relative activity regulated by the cell (coordination model)? There is evidence that the cell can indeed regulate the activity of different motors, such that different kinds of cargo get localized precisely to specific locations in the cell. This regulation, if it happens, occurs on the size scale of nanometers. We would like to understand the components, mechanisms and principles behind such regulation (see figure). We would also like to understand how each specific motor is designed in nature to allow such regulation.
Recent work from our Laboratory brings in Lipids (particularly cholesterol) as a player in controlling how motors work collectively to transport pathogens inside immune cells. Pathogens (e.g. bacteria, parasites) that can infect us are usually killed inside the cells of our immune system. The immune cells actually eat these pathogens, and first enclose them within a compartment called the “phagosome”. The phagosome is then carried by Motor proteins to an acidic execution chamber inside the cell called the Lysosome.
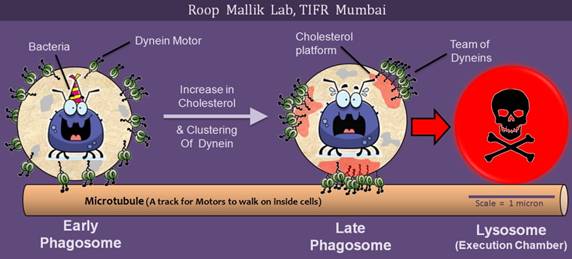
Phagosomes initially move in back-and-forth manner (see the early phagosome in above image), but then switch to almost unidirectional motion which rapidly takes them to the lysosome (red arrow for Late phagosome). This switch is aborted by several successful pathogens such as Mycobacterium Tuberculosis, Salmonella and Leishmania donovani. What switches the motion of Phagosomes, and commits them to degradation in the Lysosome? In this work we show that the switch is caused by clustering of Dynein motors into cholesterol rich platforms on the lipid membrane of a phagosome (shown with red on Late phagosome). Formation of cholesterol platforms results in assembly of a team of dyneins on the platform. The cartoon shows one such team at the bottom of the late phagosome that is rapidly transporting the phagosome (and the pathogen inside it) towards execution in the lysosome. Cholesterol, that much hated molecule, therefore shows a kinder face by helping to kill the bugs that infect us. Other motors (e.g. Kinesin) are not shown. For more details see the paper Ashim Rai et al, Cell 164, 722–734 (2016)
FURTHER READING
- Vale RD: The molecular motor toolbox for intracellular transport. Cell 2003, 112:467-480.
- Vale RD, Milligan RA: The way things move: looking under the hood of molecular motor proteins. Science 2000, 288:88-95.
- Schliwa M, Woehlke G: Molecular motors. Nature 2003, 422:759-765.
- Hirokawa N, Takemura R: Biochemical and molecular characterization of diseases linked to motor proteins. Trends in Biochemical Sciences 2003, 28: 558
- Visscher K, Schnitzer MJ, Block SM: Single kinesin molecules studied with a molecular force clamp. Nature 1999, 400:184-189.
- Mallik R, Carter BC, Lex SA, King SJ, Gross SP: Cytoplasmic dynein functions as a gear in response to load. Nature 2004, 427:649-652.
- Mallik R, Gross SP: Molecular motors: strategies to get along. Curr Biol 2004, 14:R971-982.
- M. Welte. Bidirectional transport along microtubules Current Biology, Vol 14, R525-37 (2004)
- S.P. Gross. Hither and yon: a review of bi-directional microtubule-based transport Physical Biology, Vol 1, R1-11 (2004)
- A.R. Reilein et al. Regulation of molecular motor proteins Int Rev Cytol. Vol 204:179-238 (2001)