The Cell and Tissue Engineering lab
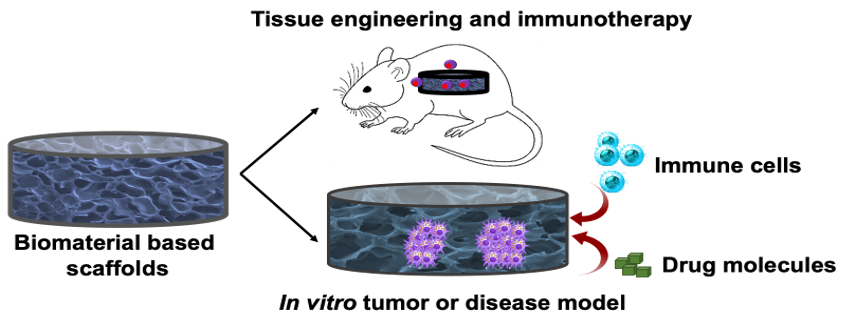
We are actively involved in designing, synthesizing, and characterizing biomaterials and scaffolds for a variety of applications, including tissue engineering, regenerative medicine, immunotherapy as well as developing in vitro 3D physiologically relevant models and biomimetic materials for studying cellular behavior. Our lab is actively developing materials for engineering immune cells for pathological conditions.
We collaborate with researchers from a variety of disciplines, including materials science, biology, chemistry, and engineering. Through interdisciplinary research, we aim to develop innovative solutions to some of the most challenging problems in tissue engineering, regenerative medicine and immunotherapy.
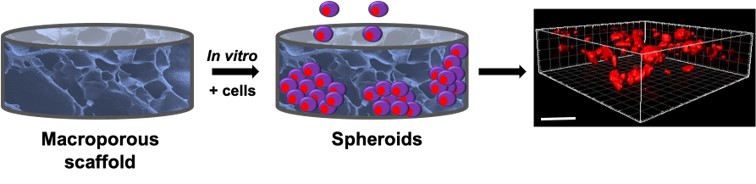
Ex vivo biomimetic studies involve culturing cells in 3D tissue models using scaffolds, enabling screening of pharmacological compounds, immunotherapeutic studies, and understanding cell-cell and cell-ECM interactions in healthy and pathological states. Scaffolds such as microporous hydrogels or macroporous cryogels have been utilized to study angiogenesis and tumor spheroid formation. The porous matrices can be tailored to mimic the tissue microenvironment by adjusting their mechanical, adhesive, and architectural properties, leading to improved clinical outcomes in bridging the gap between in vitro and in vivo animal pre-clinical studies.





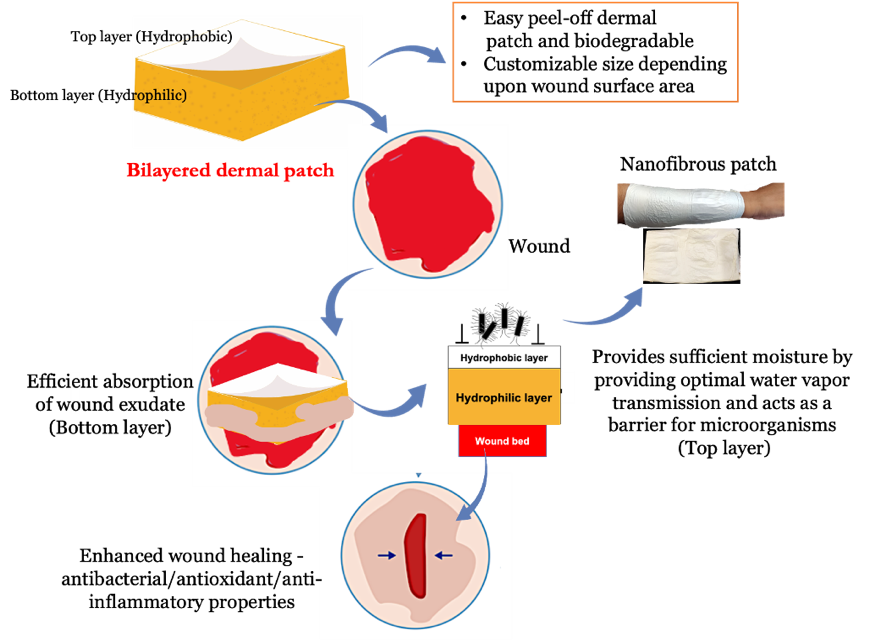
Tissue engineering and regeneration involve using 3D hydrogels, cryogels, nanofibrous, and multilayered matrices incorporating bioactive factors and/or cells to facilitate faster wound healing and tissue regeneration. Our research has resulted in the development of multifunctional bilayered skin substitutes and dermal patches for wound healing. Chronic wounds can impede the healing process due to the accumulation of inflammatory molecules and free radicals, which prevents the wound from entering the reconstruction stage. To address this, we have developed an affordable, dual-layered polymeric bandage that incorporates phytochemical agents, effectively promoting wound healing via its anti-inflammatory, antioxidant, and antibacterial properties. Studies have shown that the bilayered polymeric bandages expedite wound healing in vitro and in rat models by reducing cytokine levels released by M1 macrophages and T cells. Moreover, bilayered skin substitutes developed using polymer blends and wound healing agents can be used as epidermal or dermal equivalents, making them a promising approach for tissue engineering and regeneration.


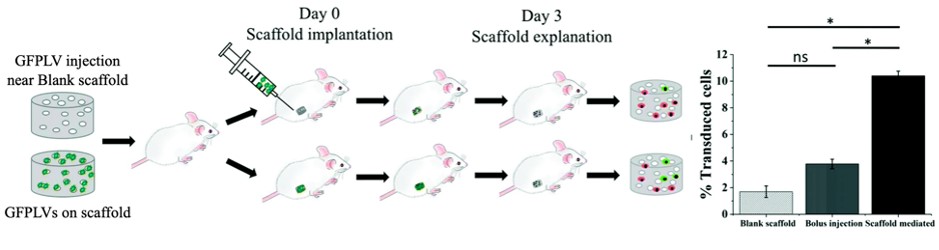
Our research has led to the development of bioactive implants that enable in situ engineering of cells and cell delivery through gene delivery. These implants offer a less invasive and cost-effective alternative to adoptive cell therapy, which is a popular cell-based immunotherapeutic strategy for cancer and autoimmune disorders. Our bioactive implants have shown promising results in generating tumor-specific T-cells for solid tumor malignancies, without the need for prolonged ex vivo manipulation of cells. Furthermore, these implants have demonstrated their ability to create a local niche that allows for efficient transduction of immune cells, leading to pre-clinical success. We are also exploring the development of less invasive implants for cell delivery and other applications.






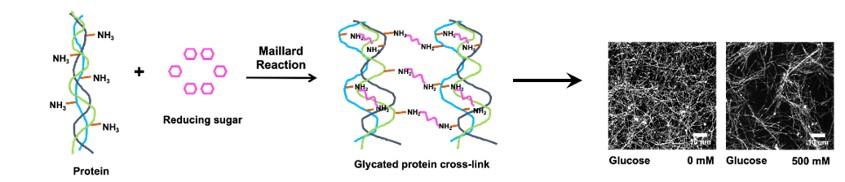
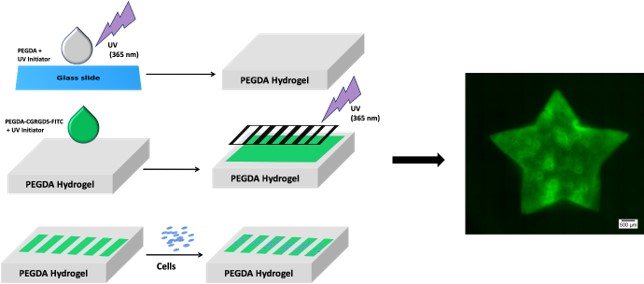
Polymers can be engineered to contain specific cell adhesive moieties, allowing for precise manipulation of the number and type of cells along a particular area and shape. This enables the systematic study of cell-cell and cell-matrix interactions, providing valuable insights into various biological processes. For instance, a collagen-based 3D matrix system has been developed to mimic the effect of increasing glucose concentration on the extracellular matrix and its subsequent impact on cells, creating a model for diabetes research. Moreover, this strategy can also be used to explore the effect of glycation on tissue-resident immune cells, facilitating the development of potential therapeutic interventions for various pathophysiological conditions.
.