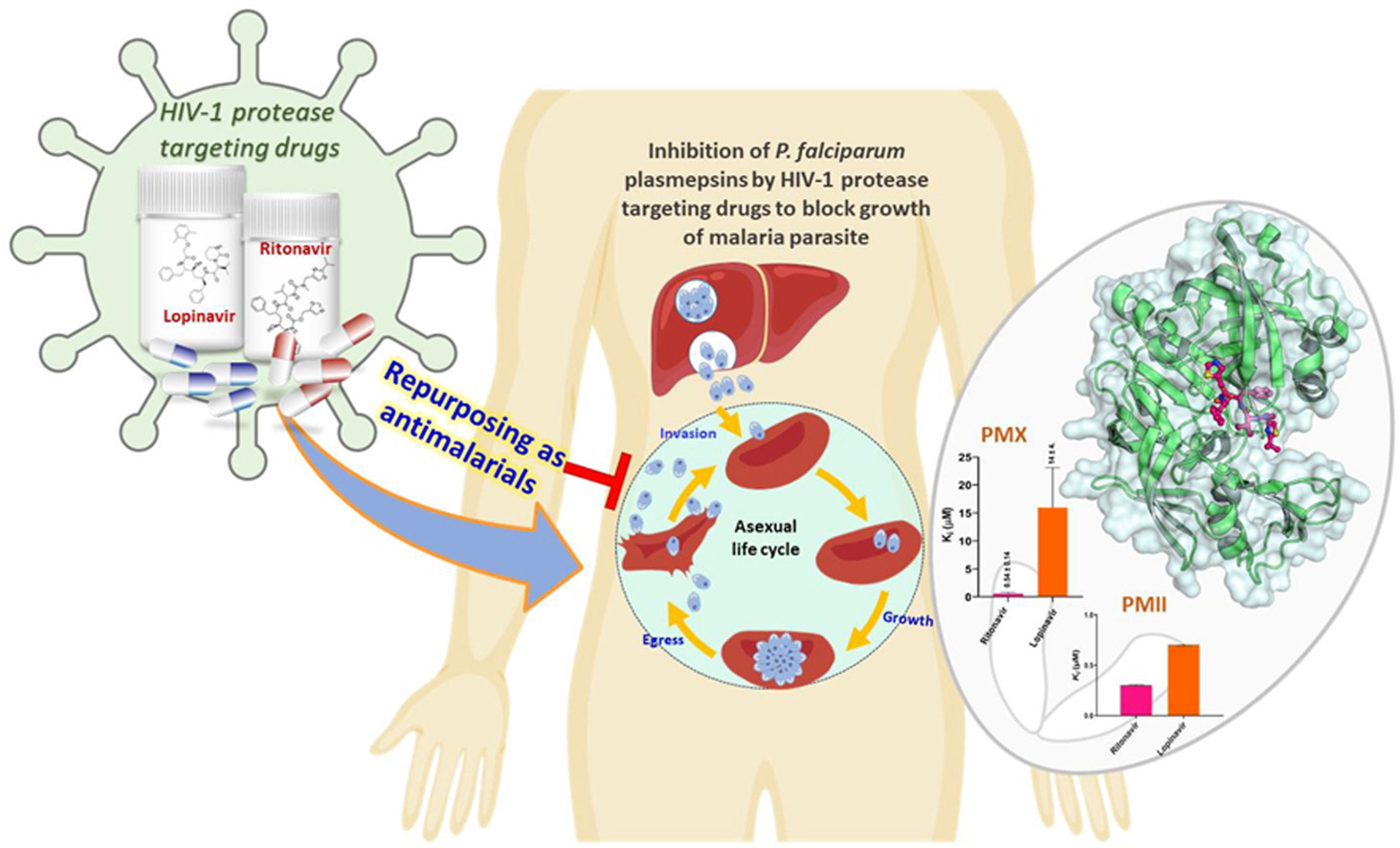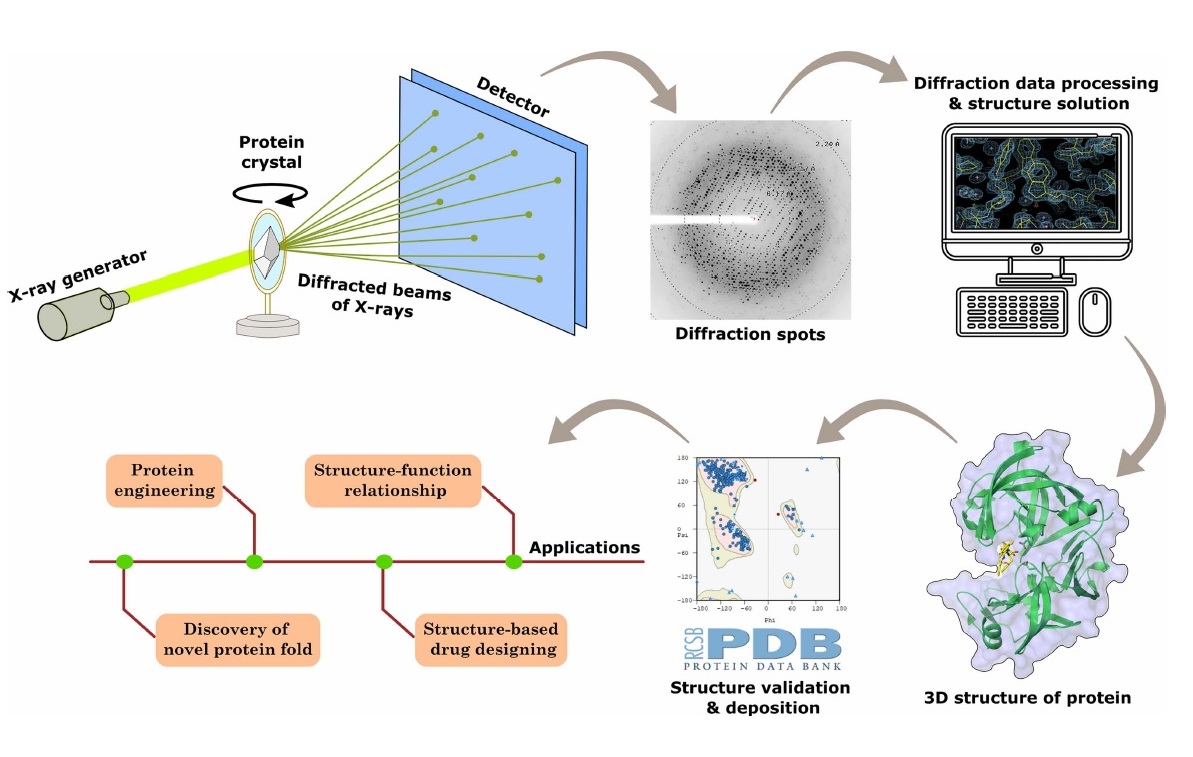
IIT Bombay
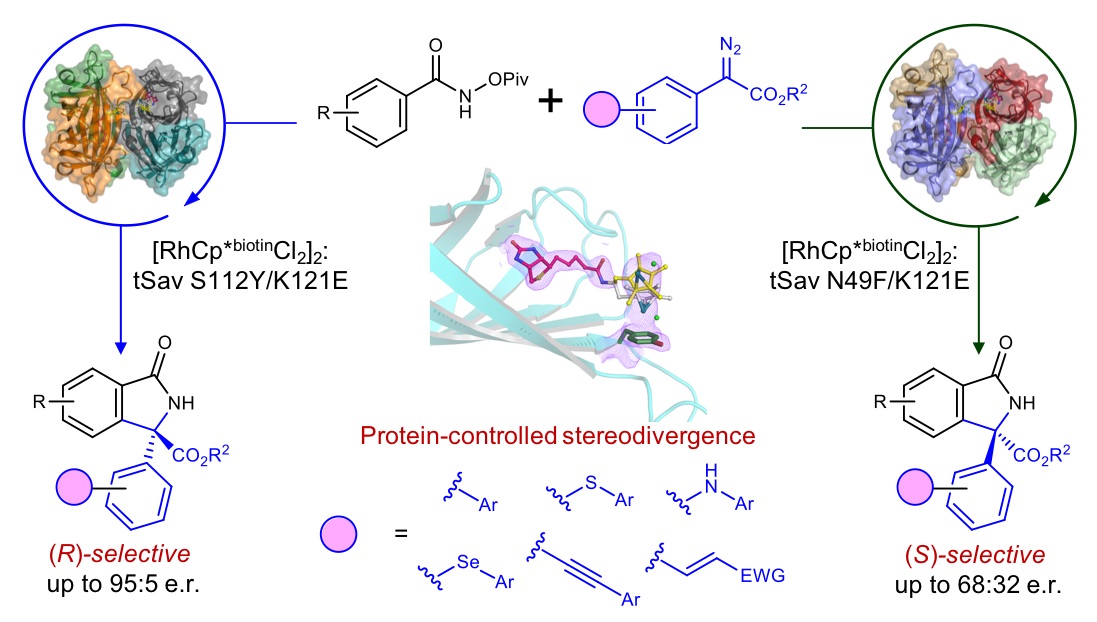
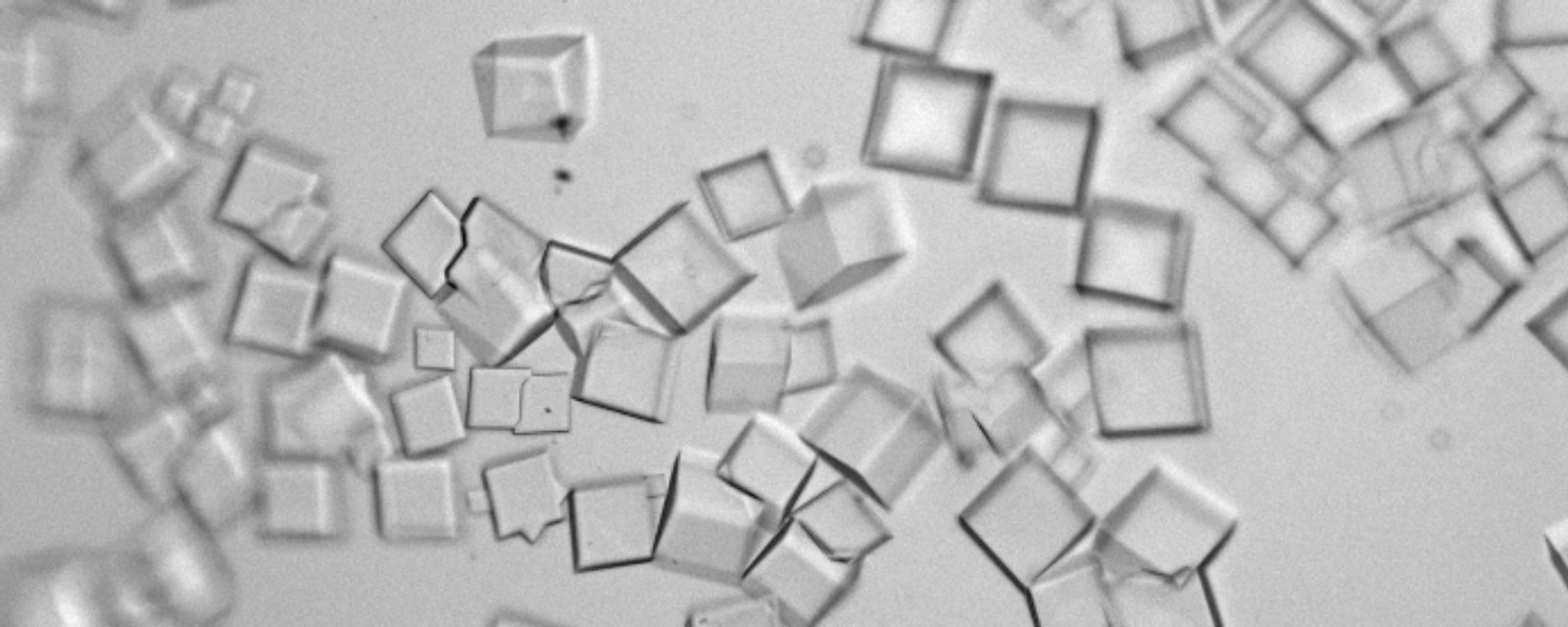
Protein Crystals

IIT Bombay
PB Group Members
IIT Bombay
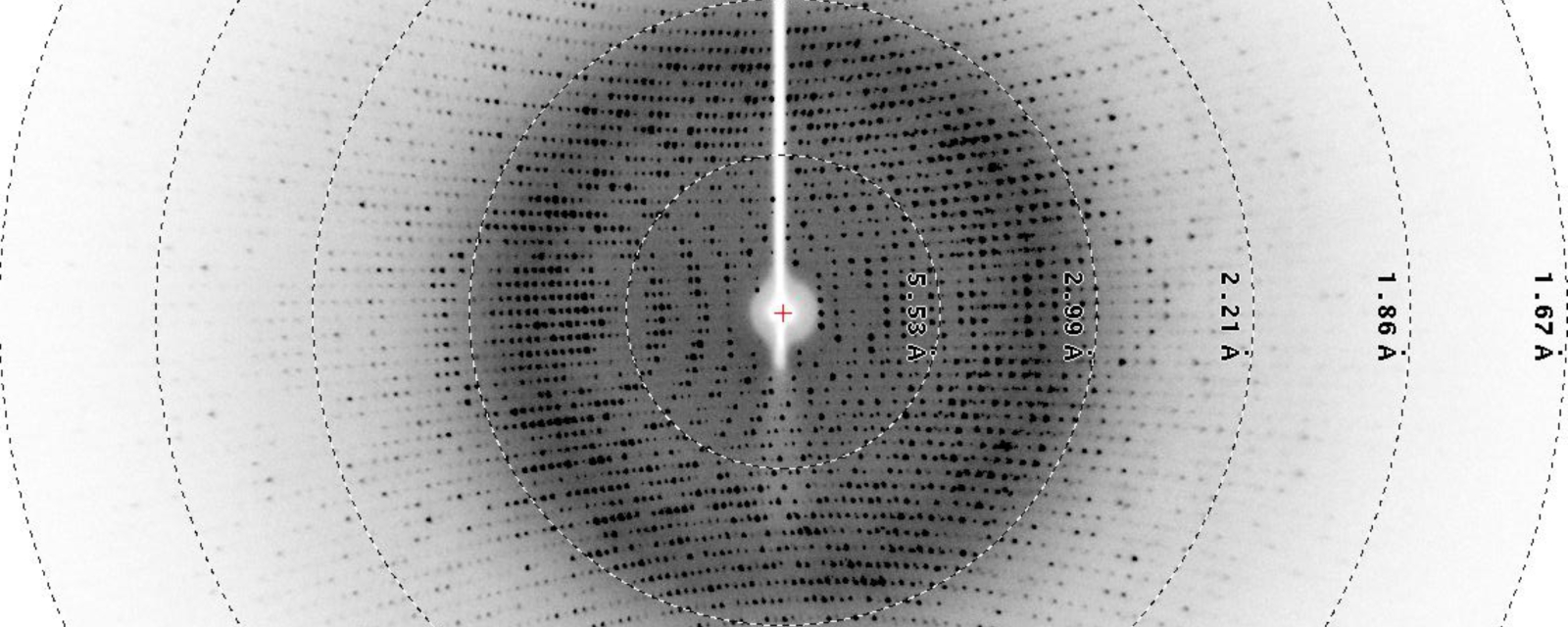
Diffraction Image

IIT Bombay
Diffactometer and Protein Crystal Mouting

IIT Bombay
Cryo-EM Grid Atlas Inspection
IIT Bombay
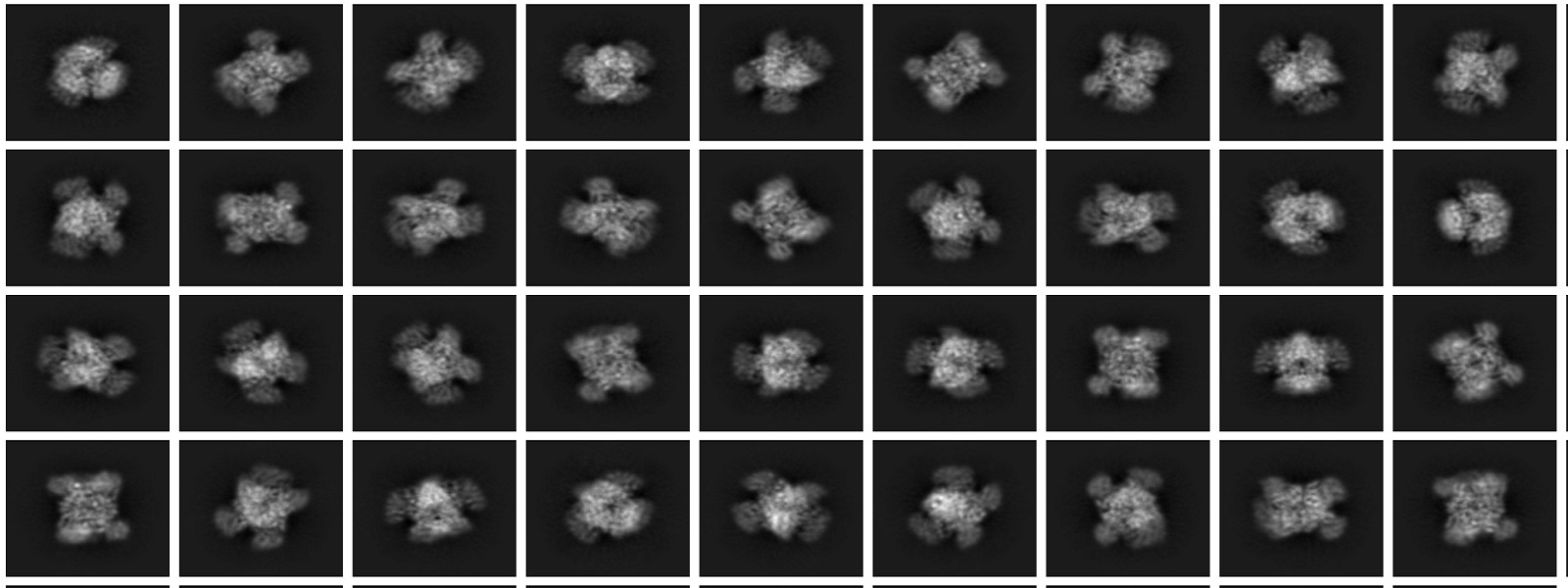
Cryo-EM Particle 2D-Classification

IIT Bombay
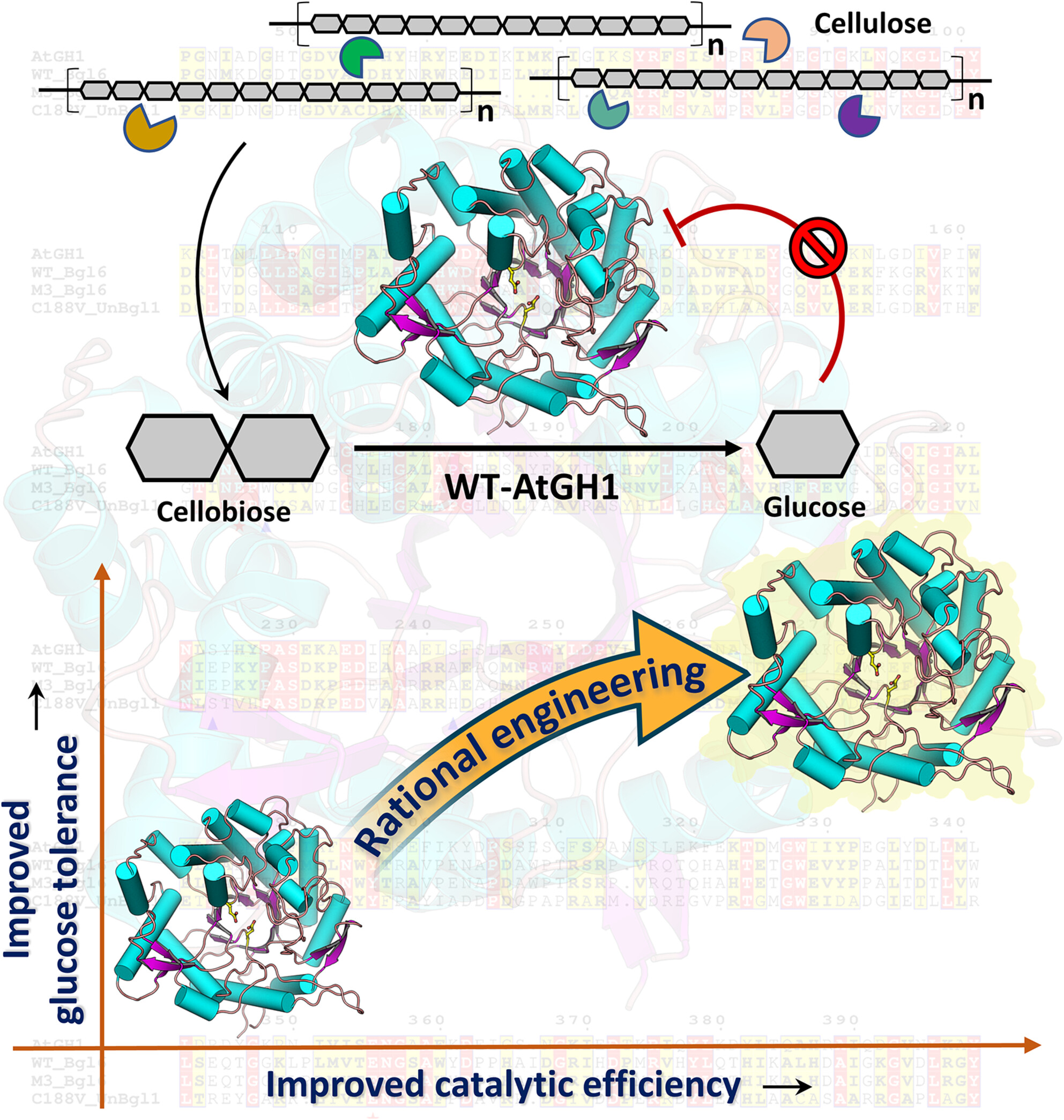
Protein Crystallization

IIT Bombay
Crystal Freezing
IIT Bombay
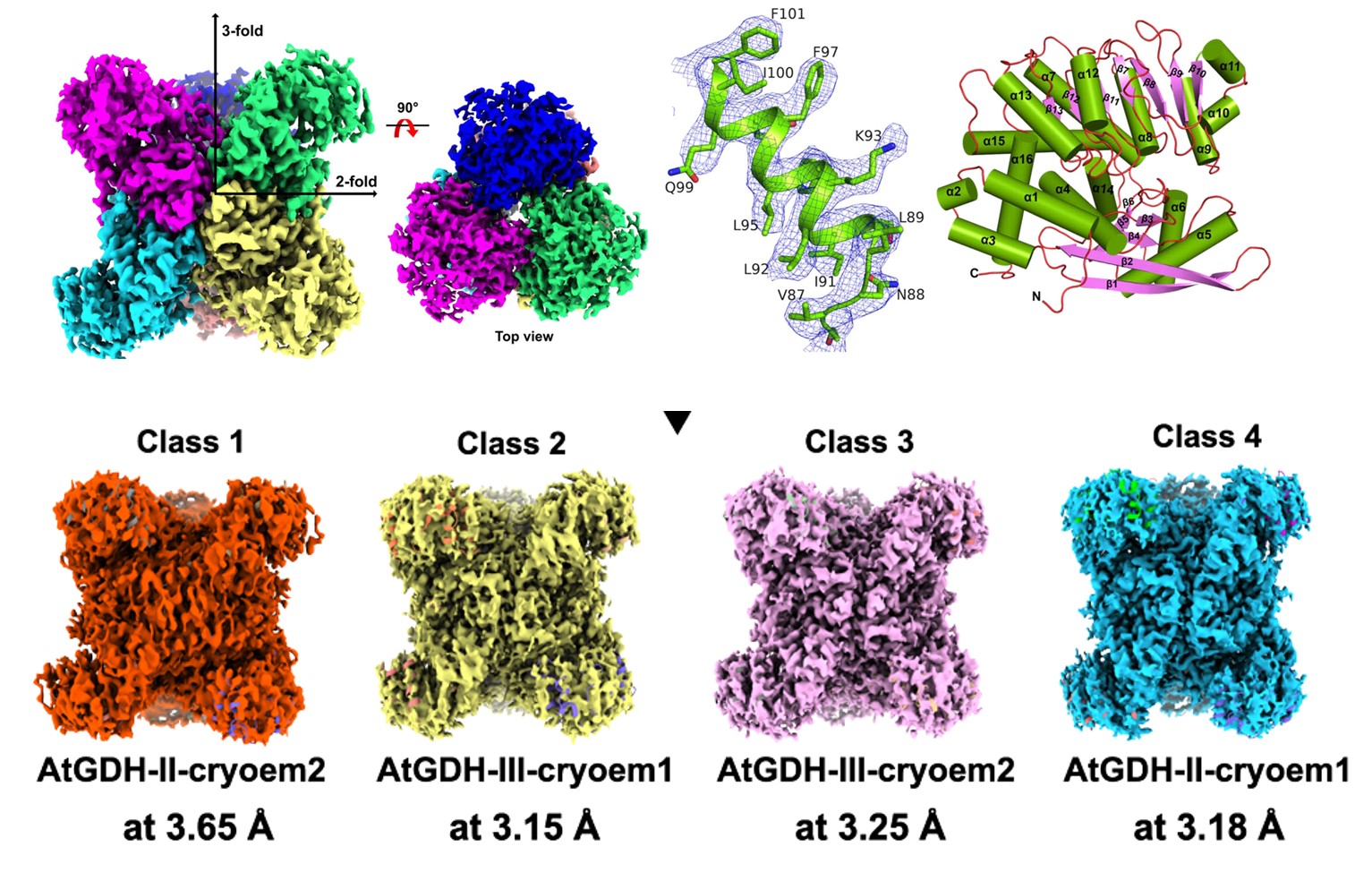
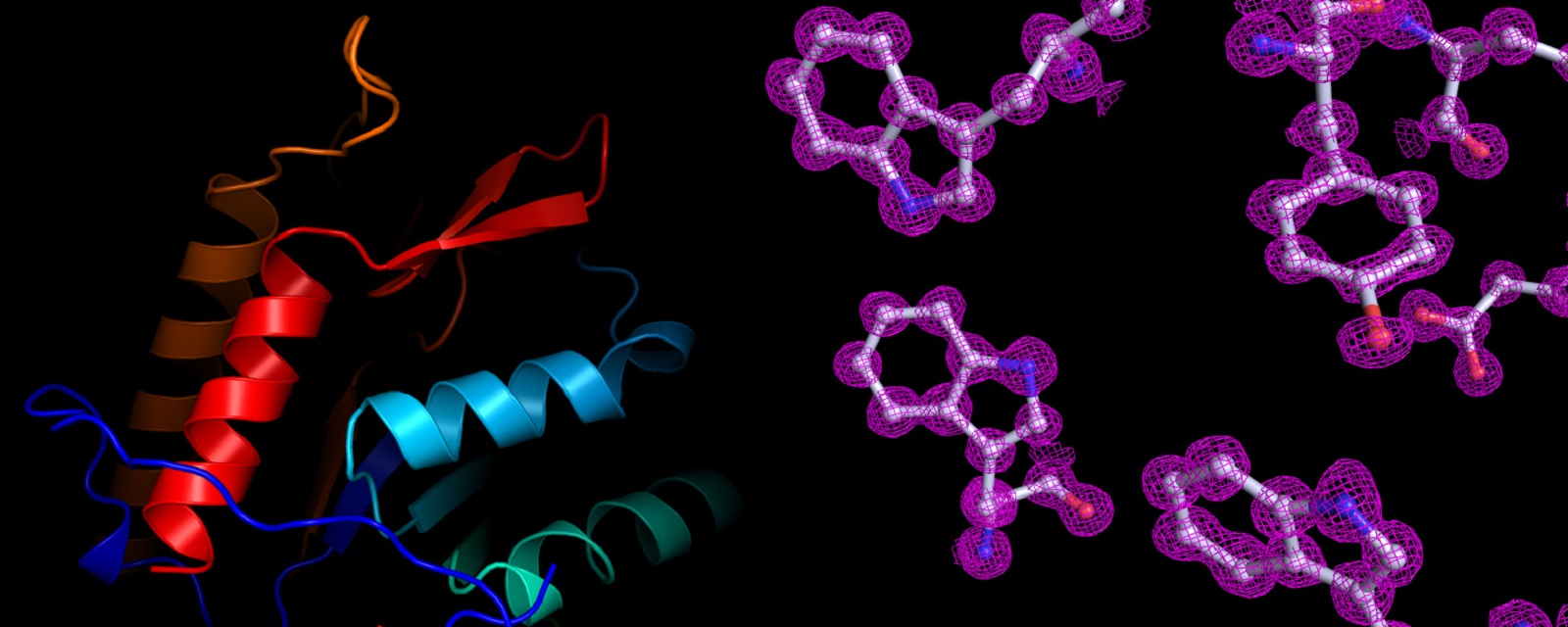
Protein Structure and Electron Density

IIT Bombay
Diffraction Data Collection at IIT Bombay

IIT Bombay
Cryo-EM Facility at IIT Bombay

IIT Bombay
Cryo-EM Data Collection
IIT Bombay
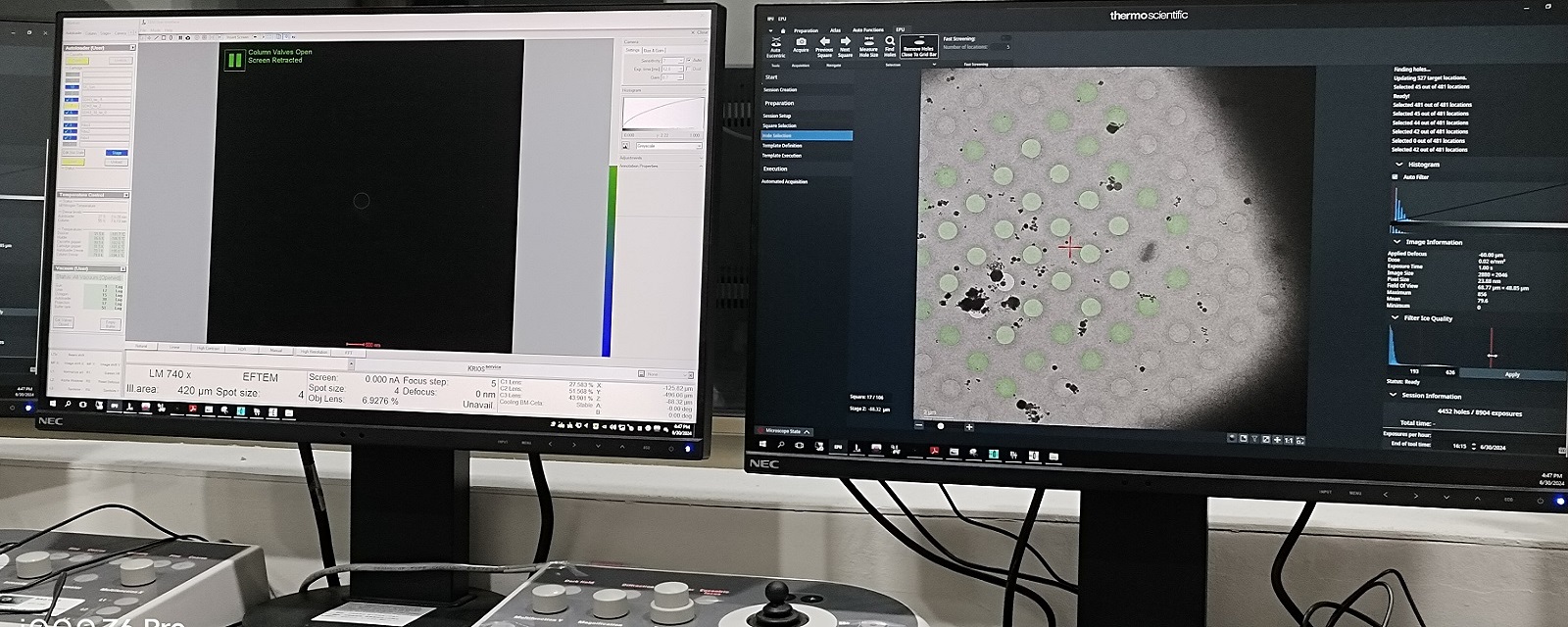
Grid Square Hole Selection
IIT Bombay
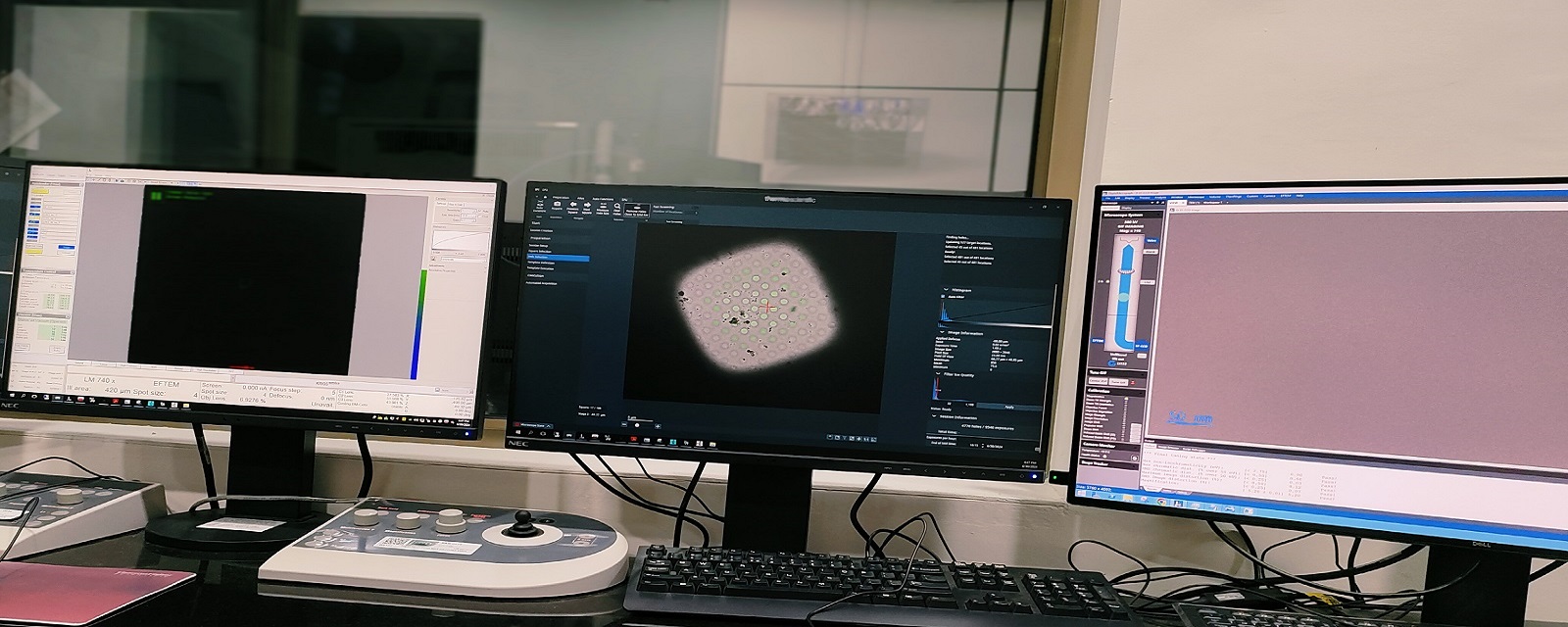
Setting up Cryo-EM Data Collection

IIT Bombay
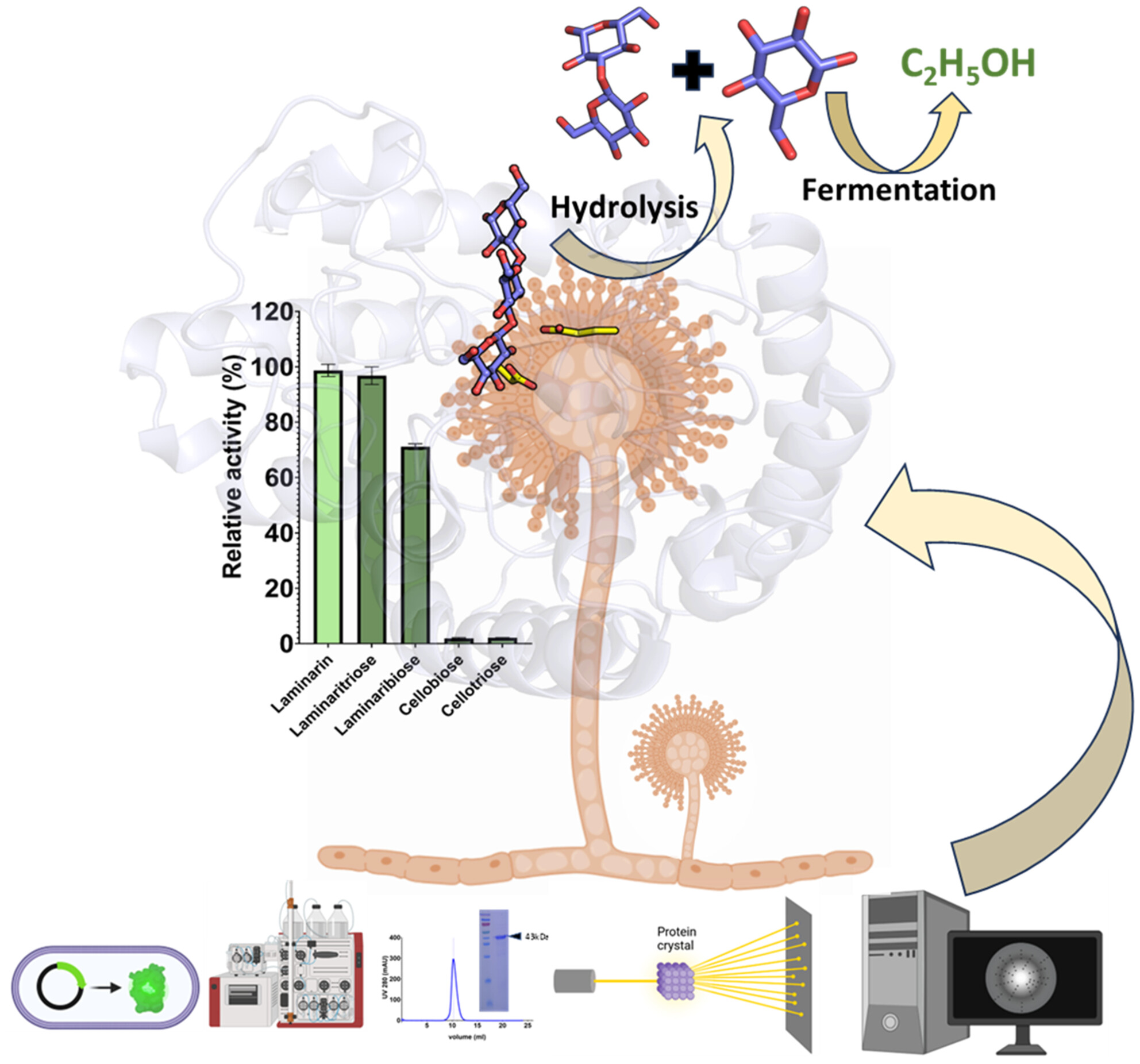
Protein Purification

IIT Bombay
Protein Crystal Freezing

IIT Bombay
FPLC Protein Purification

IIT Bombay
Protein Crystal Picking

IIT Bombay
IIT Bombay campus viewed from Sameer hill

IIT Bombay
IIT Bombay main building

IIT Bombay
BSBE building