Amyloid is the fibrillar state of polypeptide/protein aggregates with cross-β-sheet conformation. In amyloid fibrils, proteins are self-associated into nano-structures that bind dyes like Congo red and Thioflavin T. Amyloids are the major pathological hallmark of several human diseases including Alzheimer’s, Creutzfeldt-Jakob, and Parkinson’s disease. Amyloids are also associated with native biological functions such as hormone storage in secretory granules of the pituitary and Pmel17 amyloid in melanosomes in mammals. Understanding the protein aggregation and amyloid formation by proteins is thus important for designing drugs against amyloid diseases.
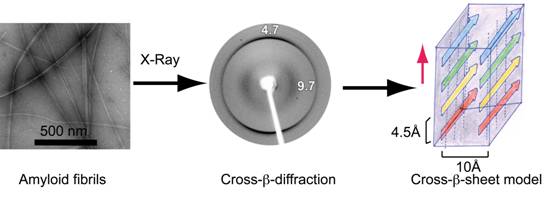
Our group is interested in studying amyloid formation by protein/peptides associated with human diseases and native biological functions (functional amyloid). The major goal of the laboratory is to study the pathway(s) of protein/peptide aggregation in general, to develop the structure-function relationship of intermediate aggregates, and designing inhibitors against amyloid aggregation. We use α-synuclein (relevant to Parkinson’s disease), Amyloid β-protein (Aβ40/42, relevant to Alzheimer’s disease), mouse prions (prion diseases), and several pituitary protein hormones (relevant to storage and pituitary carcinoma) for our studies.
The Laboratory is also interested to exploit amyloid material for drug delivery and functional bio-nano-materials applications. Please see subsequent sections for more information on different research themes pursued by the group.
Parkinson’s disease (PD) is a common neurodegenerative disorder, where the dopaminergic cells in the individual’s midbrain are affected. The common clinical symptoms of PD include resting tremor, rigidity, slowing of movement, postural instability. The major pathological hallmark of the disease is the accumulation of the protein α-synuclein in Lewy bodies (LB) and Lewy neuritis (LN) in the dopaminergic neurons in the substantia nigra region. PD is considered mostly as sporadic (no definitive causes are known). However, it is believed that the disease may be caused by multiple factors. One of the most potent causes of PD is considered to be the accumulation of the protein α-synuclein into toxic oligomers and fibrils in dopaminergic neurons that are supposed to synthesize dopamine. The exact mechanism and factors leading to aggregation of α-synuclein are not clear yet. Apart from sporadic cases of PD, some rare cases of individuals get afflicted with PD due to α-synuclein mutation (a familial form of PD), where many individuals develop the disease at an early stage in their lives. In PD, all current treatments are mostly symptomatic and there is no specific drug, which can cure this disease.
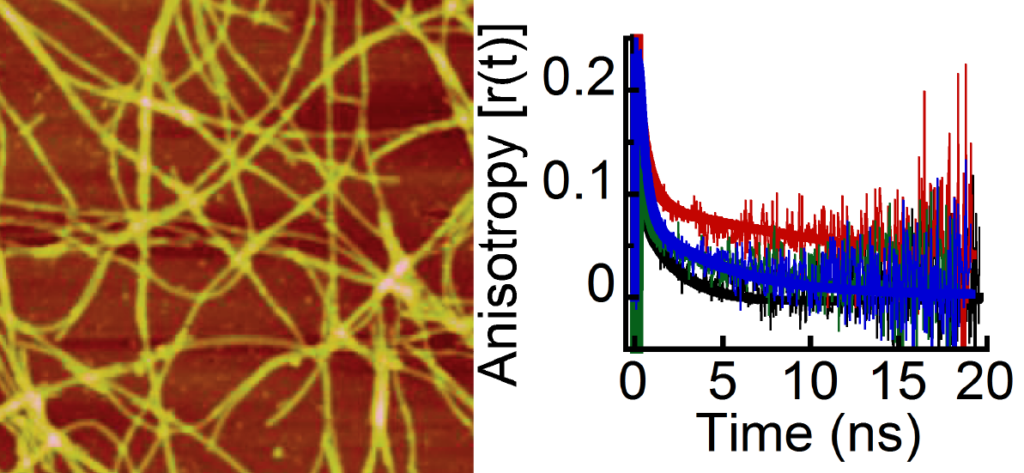
Many previous studies including in vitro, in the cell, and animal models have clearly suggested that α-synuclein aggregation and amyloid formation is one of the potential causes for dopaminergic neuron degeneration. Recent studies, however, have suggested that α-synuclein oligomers formed during aggregation are the most toxic species responsible for dopaminergic neuron loss occurring in the PD. Therefore understanding and establishing detailed pathways on α-synuclein accumulation and their structure-toxicity relationship will be of significant help in PD therapy. In our laboratory, we express and purify human α-synuclein in E. coli bacteria and model the α-synuclein accumulation in vitro. The advantage of this approach is that we can simultaneously monitor how structural and morphological transitions accompany the protein aggregation and amyloid formation of α-synuclein. We are also studying how different familial mutations affect the α-synuclein structure, both in soluble form as well as fibrillar form, which may contribute to the disease phenotypes. We are also studying α-synuclein accumulation in cells and Drosophila melanogaster to understand the role of α-synuclein aggregates in PD.
Research members- Surabhi, Soumik, Laxmikant, Ajay, Arunima.
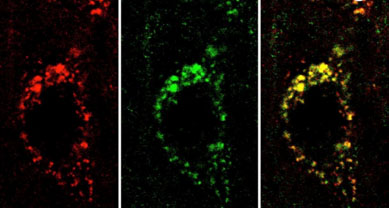
Amyloids are not only involved in various human diseases, but they can also perform native biological functions of the host organism (functional amyloid). Examples include curli amyloid fibrils in E. coli which help bacteria for their surface adhesion, biofilm formation, and colonization. Pmel17 amyloids inside melanosomes are shown to template/facilitate melanin synthesis effectively. Recently peptide/protein hormones have also been shown to be stored in amyloid form inside the secretory granules of the pituitary, which effectively help long-term storage of regulated secretory protein inside the cells. Our lab is currently studying the possible role of protein aggregation and amyloid formation by various protein/peptides for their secretory granule (SG) biogenesis. We study in vitro aggregation and amyloid fibrillation of these proteins/peptide hormones using various biophysical methods including fluorescence spectroscopy, electron/atomic force microscopy, and NMR spectroscopy to develop structure-function of protein for secretory action (both storage and release). We also frequently use various animal cells and tissues to study the storage state of protein/peptides. Detailed understanding of the functional amyloid formation of hormones may provide the mechanism of secretory granule biogenesis and associated human diseases with defective hormone storage and secretion.
Research members- Debdeep, Semanti, Namrata.
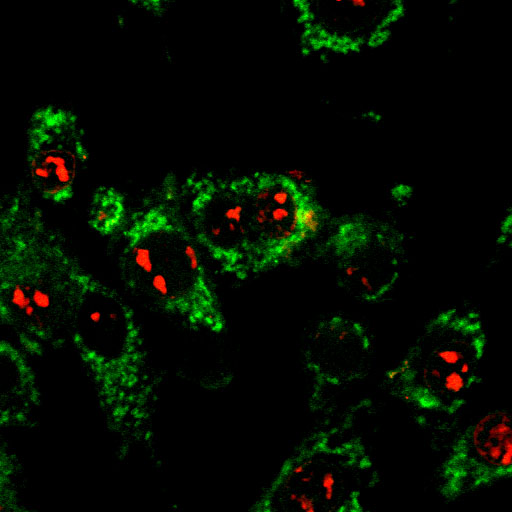
The transcriptional regulator p53 plays an essential role in tumor suppression. p53, in its active tetrameric form, suppresses cell cycle progression or induces apoptosis in response to DNA damage. Most of the cancers are often characterized by the loss of function of p53 that frequently accumulates in the nucleus as well as in some cases in the cytoplasm. We hypothesize that aggregation and amyloid formation of p53 in the cytoplasm and/or nucleus could result in loss of p53’s function as a tumor suppressor. In our lab, we study the structure-aggregation-function relationship of wild-type and mutant p53 in vitro and in cultured mammalian cells. We also study the aggregation of p53 and their functional relevance in transcription using S. cerevisiae (yeast) as a model system. We are also interested in analyzing the prion-like behavior of p53 aggregates in mammalian cells as well as in yeast.
Research members- Ambuja, Debalina, Ajoy, Satya, Shinjini.
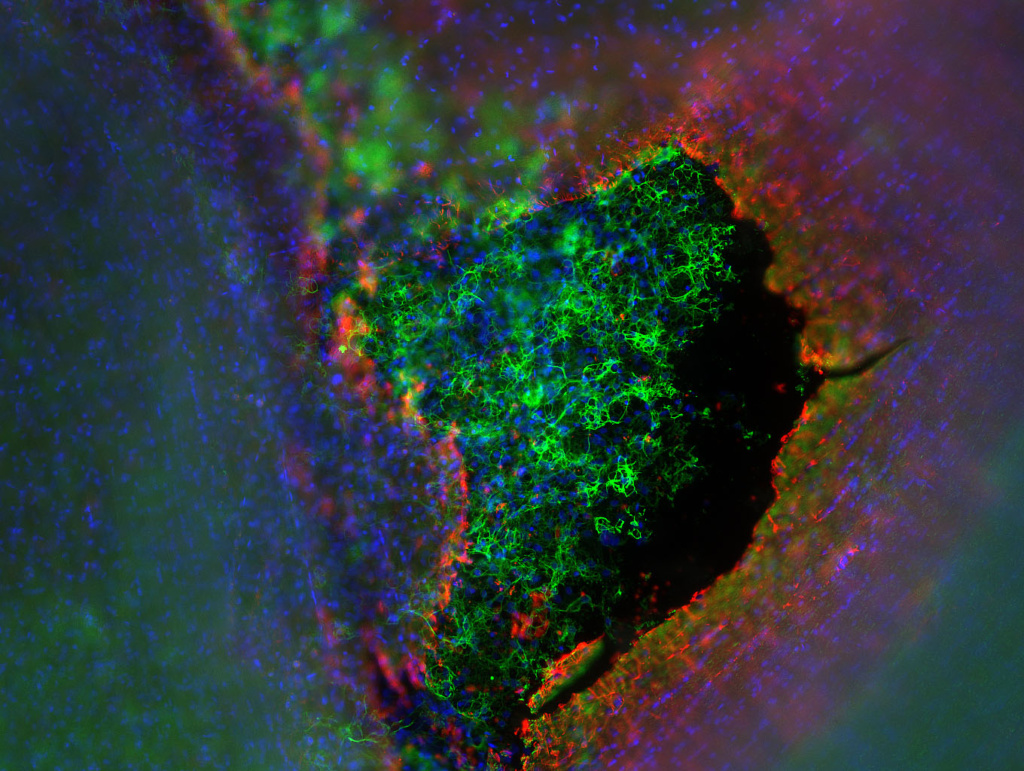
Although amyloids consist of highly ordered protein/peptide aggregates and are associated with diseases, they can also perform native biological functions for the host organism (functional amyloid). Along with the functional role of amyloids, their highly repetitive structure, stability, resistance against harsh physical and chemical conditions like temperature, extreme pH, and proteases, makes them attractive for designing smart biomaterials. The unique surface properties, consisting of a combination of charged and hydrophobic patches, enables them to bind to various small molecules and large macromolecules/polymers. Many microorganisms also use amyloids for their surface attachment and colonization and biofilm formation. Based on these properties, we are actively engaged in designing amyloid-based biomaterials for various applications including tissue engineering and stem cell differentiation. In the tissue engineering front, we are developing smart materials for neural regeneration of the central nervous system.
Research members- Komal, Pradeep, Manisha, Namrata.
